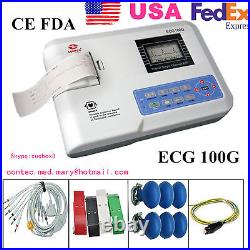











FDA Portable Digital 1-channel Electrocardiograph ECG Machine EKG, 12 leads, USA. Model:ECC100G+ONE printer paper. The monitor is registered on the Australian. Register of Therapeutic Goods (ARTG) with the code 197923. ECG100G is a kind of single channel electrocardiograph, which features in printing ECG waveform with thermal printing system, prompting for “Lead off” or “Lack of paper”, multi-language interface, recording ECG waveform with manual or auto mode, convenient to operate. 2.7 STN-LCD, accurate and clear ECG waveform and working state display, economical to save recording paper. Touch keyboard, simple and convenient to operate. Adopt digital signal processing technology to get high-quality ECG waveforms via AC filter, baseline filter and EMG filter of ECG signal. Real-time record single-channel ECG waveform and annotationincluding lead mark, sensitivity, paper speed and filter state, etc. Adopt high-resolution thermal printing system(8 dot/mm), no need for any adjustments. Recording frequency: up to 150Hz. In AUTO mode, finish all operation by pressing the button once, which improves work efficiency. Multi-language (Chinese, English, French, Italian, Turkish, Spanish and German) interface and report. AC/DC, built-in rechargeable lithium battery. In optimal DC state, up to 7h standby time, continuous print up to 4h, record up to 160 ECG waveform. Temperature: 5? 40????? Relative humidity: 25%80%(non-condensing)???? Atmospheric pressure: 700hPa1060hPa. Transport and storage environment. Temperature: -40? +55????? Relative humidity:? 95%???? Atmospheric pressure: 500hPa1060hPa. Input mode: floating and defibrillation protection. Frequency response: 0.05Hz 150Hz(-3dB+0.4dB). CMRR: >60dB, >100dB(add filter). Time constant:? 3.2s. Sensitivity: 5, 10, 20 mm/mV ±5%, standard sensitivity: 10mm/mV±2%. Input circuit current:? 50nA. Safety classification: class? , type CF and defibrillation-proof applied part. Recording mode: thermal printing system. Paper size: 50mm(W)×20m(L). EMG interference filter: 35Hz(-3dB). AC filter: 50Hz/60Hz(-20dB). Paper speed: 25, 50mm/s, ±5%. AC: 100V240V(50/60Hz)???? DC: 7.4V/2000mAh rechargeable lithium battery. Fuse specification: two AC time lag fuse(? 5×20mm), T1.6AL250V. Working mode: continuous working. Dimension: 315mm(L)×215mm(W)×77mm(H). A thermal recording paper. It take 7-20 bussiness days to arrive you. EMS, UPS, TNT and DHL could be used for specail requirment if you agree to pay more. The sale of this item may be subject to regulation by the U. Food and Drug Administration and state and local regulatory agencies. If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA’s Center for Devices and Radiological Health. The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923. And certified by FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved. Contec Medical Systems Skype:xuebox3.


